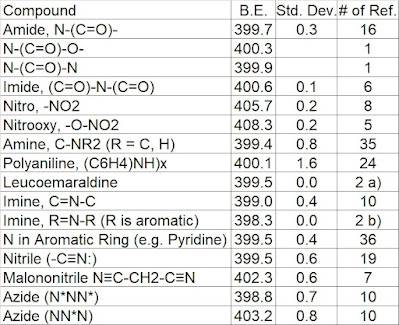
 |
| Table 1. Summary of N 1s binding energies [1,2]. a) 399.4 -399.6 eV found for leucoemaraldine [3] b) 398.3 to 398.5 eV found for emeraldine/pernigraniline [3] |
Other species of interest:
Isocyanate (R-N=C=O), 400.0 eV [4] (from bulk methylene diphenyl di-isocyanate)
References:
|
[1] C.D. Wagner, A.V. Naumkin, A. Kraut-Vass, J.W. Allison, C.J. Powell, J.R.Jr. Rumble, NIST Standard Reference Database 20, Version 3.4 (web version) (http:/srdata.nist.gov/xps/) 2003.
[2] G. Beamson, D. Briggs, High Resolution XPS of Organic Polymers - The Scienta ESCA300 Database Wiley Interscience, 1992.
[4] S. Tardio, M.-L. Abel, R.H. Carr, J.F. Watts, Int. J. Adhes. Adhes. 88 (2019) 1-10.
|




